1.5 States of Matter Study Guide
- Due Feb 25, 2022 at 11:59pm
- Points 24
- Questions 12
- Available until Mar 17, 2022 at 11:59pm
- Time Limit None
- Allowed Attempts 5
Instructions
1.5 States of Matter (8.1.5)
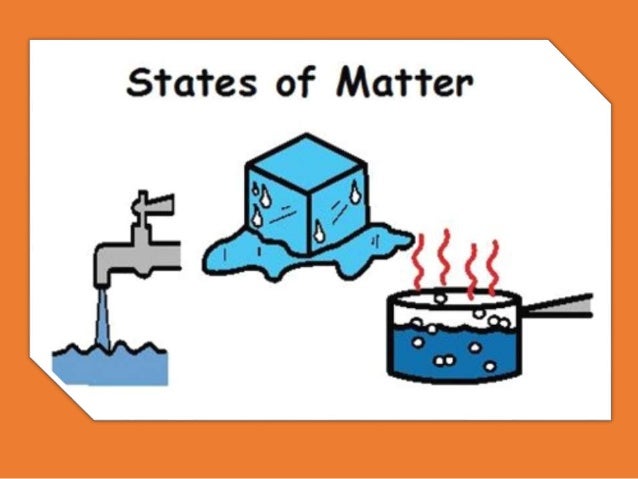
Explore this Phenomenon
- What observations can you make about the pictures and how the pictures are
- connected?
- What questions can you ask about these different pictures?
- How can you explain the differences in the pictures?
8.1.5 The States of Matter
Develop a model that uses computational thinking to illustrate the cause and effect relationships in particle motion, temperature, density, and state of a pure substance when heat energy is added or removed. Emphasize molecular-level models of solids, liquids, and gases to show how adding or removing heat energy can result in phase changes and on calculating density of a substance’s state. (PS3.A)
In this section, focus on cause and effect. Observe the effect that adding or removing heat causes on the particle motion, density, and state of pure substances.
Matter and Its States
Matter typically exists in one of three states: solid, liquid, or gas. The state of matter of a substance is one of its properties. Some substances exist as gases at room temperature (oxygen and carbon dioxide), while others, like water and mercury metal, exist as liquids . Most metals exist as solids at room temperature. Most elements can exist in any of these three states.
Note: Technically speaking a fourth state of matter called plasma exists, but it does not occur often on earth, so we will omit it from our study here.
There are three states of matter that are commonly found on Earth:
Solid, Liquid, Gas
Solids: have a definite shape and definite volume
- particles are not allowed to move around freely
- particles only vibrate in place, attracted and held to each other


Liquids: have a definite volume, but not a definite shape
- takes the shape of the container
- particles vibrate, and can also slide around each other
- particles are still attracted to each other
Gas: no definite shape and no definite volume
- particles move around freely, with little to no attraction to each other
- takes the shape AND the volume of the container

*For MOST substances, gas is less dense than liquid, which is less dense than solid*

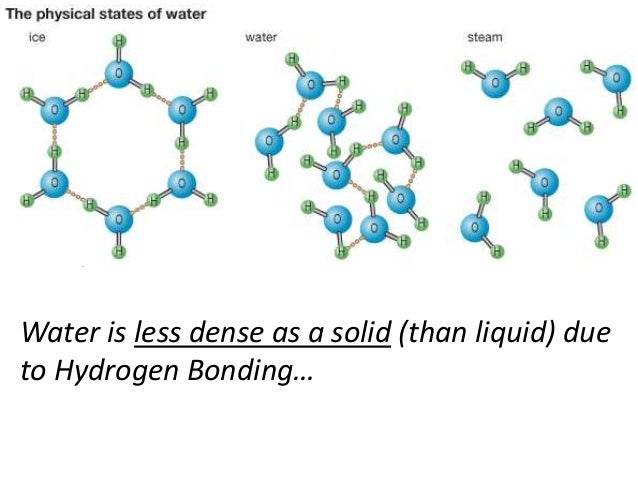
*Water is an exception: solid water is LESS dense than liquid water*
Changes of State
- Each state is different because the particles have different amounts of energy
- more energy = more movement
- gases have the most energy (move the most freely)
- liquids have more energy (slide around) than solids (just vibrate)
- Changing between states requires the particles to gain or lose energy, in the form of heat
Evaporation: changing from liquid to gas
-
-
- adding heat to liquid gives particles more energy, until they move freely as a gas
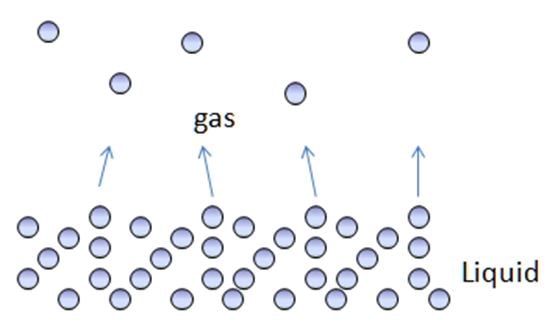
- adding heat to liquid gives particles more energy, until they move freely as a gas
-
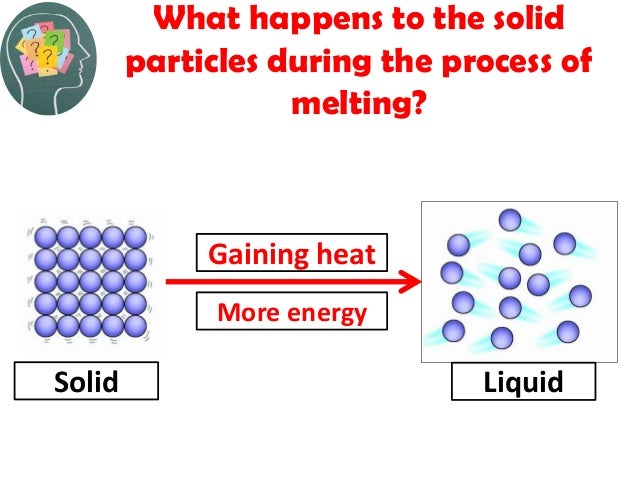
Melting (aka Liquification): change from solid to liquid
-
-
- adding heat to solid gives particles more energy, until they can slide around more
-
Freezing (aka Solidification): Change from liquid to solid
- Removing heat from liquid causes particles to lose energy, and stop sliding around
Condensation: change from gas to liquid
-
-
- Removing heat from gas causes particles to lose energy, and move less freely
-

Sublimation: change from solid, skipping liquid, directly to gas

-
- adding heat to solid gives particles more energy, until they move freely as a gas
Deposition: change from gas, skipping liquid, directly to solid
-
-
- Removing heat from gas causes particles to lose energy, and move less freely
-

How is heat energy added or removed?
- First, Heat is a type of energy (aka: Thermal Energy)
- It is the energy of particles moving
- Temperature is a measure of heat energy
- There is NO SUCH THING as Cold.
- Cold is just the absence of heat energy
- Second, Heat is added or removed from a substance because of the surrounding environment
- Heat energy always moves from substances with more heat, to substances with less heat
- FROM WARMER TO COOLER SUBSTANCES!
- Heat energy always moves from substances with more heat, to substances with less heat
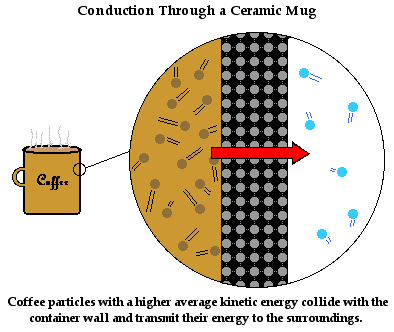
**Cooling a substance is not adding cold to it, its removing heat from it**
- The inside of a fridge is cool, so heat moves from the food to the air in the fridge, until they reach the same temperature
Let’s do a quick review of the states of matter. Solids have a definite shape (rigid), a definite volume, and the particles are not free to move very much other than just vibrate. Liquids have no definite shape (take the shape of the container up to the limit of the volume), though they do have a definite volume, and the particles are free to move over each other but are still attracted to each other. Gases have no definite shape (take the shape of the container), have no definite volume, and the particles move in random motion with little or no attraction to each other.
The Role of Energy in Changes of State
Suppose that you leave some squares of chocolate candy in the hot sun . A couple of hours later, you notice that the chocolate has turned into a puddle like the one pictured in the photo.
In order for solid chocolate to melt and change to a liquid, the particles of chocolate must gain energy . The chocolate pictured in the photo gained energy from sunlight. When matter changes from one state to another, it either absorbs energy as when chocolate melts—or releases energy--as when water freezes. For example, if you were to place the melted chocolate in a refrigerator, it would lose heat energy to the cold air inside the refrigerator. As a result, the liquid chocolate would change to a solid again. When matter changes from one state to another, it is referred to as a phase change. When liquid water turns to ice, it has a phase change from liquid to solid.
The Effects of Adding or Removing Energy
Adding energy is a “cause” that has multiple “effects”. As discussed above, adding heat energy often causes phase changes. Phase changes happen when heat energy is added because heat energy causes particle motion to increase. As particles move faster the space between them increases, which often causes a phase change. The more space that forms between particles will lower the substance’s density. These effects can be seen also when energy is removed but the effects go in the opposite direction. When energy is removed particle motion decreases and the space between particles decreases. When energy is added or removed the motion of particles and distance between particles changes, but the particles themselves do not change.
Defining Density
Density is an important property of matter. It reflects how closely packed the particles of matter are. When particles are packed together more tightly, matter has greater density. Differences in density of matter explain many phenomena. For example, differences in density of cool and warm ocean water explain why currents flow through the oceans.
To better understand density, think about a bowling ball and volleyball. Imagine lifting each ball. The two balls are about the same size, but the bowling ball feels much heavier than the volleyball. That’s because the bowling ball is made of solid plastic, which contains a lot of tightly packed particles of matter. The volleyball, in contrast, is full of air, which contains fewer, more widely spaced particles of matter. In other words, the matter inside the bowling ball is denser than the matter inside the volleyball.
A bowling ball is more dense than a volleyball. Although both balls are smaller in size, the bowling ball feels much heavier than the volleyball.
Calculating Density
As stated, the density of matter is the amount of matter in a given space. The amount of matter is measured by its mass, and the space matter takes up is measured by its volume. Therefore, the density of matter can be calculated with this formula:
Density = mass
volume
For example, a book with a mass of 500 g and a volume of 1000 cm 3 , the density
would be calculated as follows:
Density = 500g = 0.5 g/cm3
1000 cm3
Q: What is the density of a liquid that has a mass of 300 g and a volume of 30 mL?
A: The density of the liquid is: Density=300 g/30 mL=10 g/mL
For information on how to calculate the density of a gas, go to the following video link. http://go.uen.org/aZg
To practice and learn more about phase changes, adding or removing energy and
figuring out density, go to this digital lab link: http://go.uen.org/b03
Putting It Together
- Explain how your understanding of what these pictures show has changed.
- Think of another phenomenon that applies to phase changes.
- Explain what is going on in the pictures based on what you have learned in this section.