1.2 Properties of Matter Study Guide
- Due Feb 11, 2022 at 11:59pm
- Points 26
- Questions 13
- Available until Mar 17, 2022 at 11:59pm
- Time Limit None
- Allowed Attempts Unlimited
Instructions
1.2 Properties of Matter (8.1.2)
Explore this Phenomenon
These objects are made of two different materials, plastic and steel.
- What are some observations you can make about these two materials?
- What is similar between them?
- What is the difference between them?
- What can each item be used for?
- What questions could you ask about why these substances are used for different things?
8.1.2 Properties of Matter
Obtain information about various properties of matter, evaluate how different materials’ properties allow them to be used for particular functions in society and communicate your findings. Emphasize general properties of matter. Examples could include color, density, flammability, hardness, malleability, odor, ability to rust, solubility, state, or the ability to react with water. (PS1.A)
In this section focus on structure and function. Structures can be designed to serve particular functions by taking into account properties of different materials and how materials can be shaped and used .
What is Matter?
Legos, steel cable, the air you breathe, the water you drink--all of it is considered matter. So is the ground beneath your feet. In fact, everything you can see and touch is made of matter. The only things that aren’t matter are forms of energy, such as light and sound. Although forms of energy are not matter, the air and other substances they travel through are. So what is matter? Matter is defined as anything that has mass and volume. Mass is the amount of matter in a substance or object. The amount of space matter takes up is its volume.
Matter may be solid, liquid, or gas.
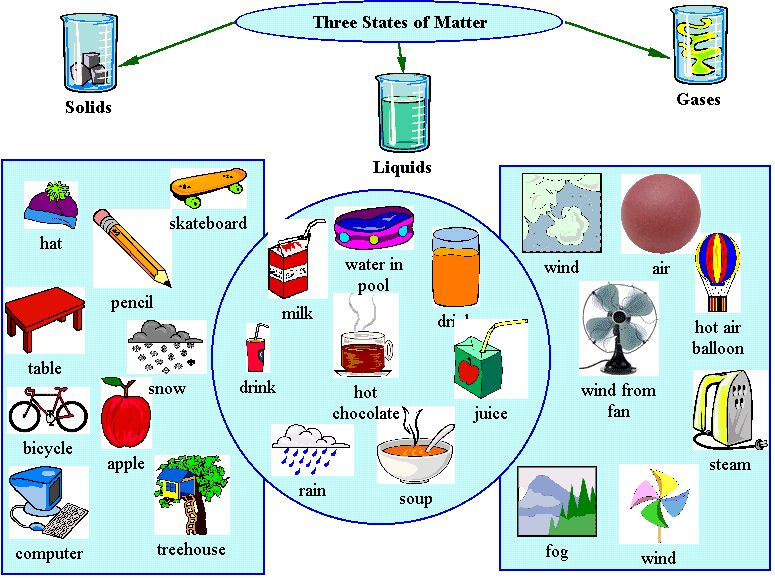
Properties of Matter
Look at the picture of the Statue of Liberty. Describe it in as many ways as possible. The things you described are called properties; they are the characteristics of matter.
Properties are separated into two different categories. Properties are classified as either chemical properties or physical properties. If you were to describe an object to someone who cannot see or feel the object you would describe the object's properties. Below is a list of some properties you could use to describe matter.
- Hardness: Whether or not an object can be scratched by something else. For example, a diamond is the hardest mineral found on Earth and can scratch most everything else. Talc is the softest mineral; it can be scratched by a fingernail.
- State of matter: Whether it is a solid, liquid, or gas.
- Melting and boiling point: This is the temperature at which a substance goes from a solid to a liquid or a liquid to a gas. For example, antifreeze has a higher boiling point and lower freezing point than water, which is useful in a car’s engine to keep it from freezing in cold weather or overheating in hot weather.
- Ability to conduct heat or electricity: Some materials conduct electricity and others do not. Aluminum and copper are good conductors, wood and plastic are not.
The plastic and the aluminum in the kettle conduct heat differently just as the copper in the wires and the plastic on the outside conduct electricity differently.
- Ability to dissolve in other substances: Some substances dissolve and others do not. Sand does not dissolve in water, sugar does.
- Density: How closely packed the atoms of matter are. A solid rock is denser than water and will sink while wood is less dense than water and will float.
- Flammability: The ability of matter to burn. Wood is flammable; iron is not. This is a chemical reaction and so makes new substances like ash, carbon dioxide, and water.
- Reactivity: The ability of matter to combine chemically with other substances. Iron is highly reactive with oxygen. When it combines with oxygen, a reddish powder called rust forms. Rust is not iron but an entirely different substance that consists of both iron and oxygen.
- Malleability: The ability of a solid to bend or be hammered into other shapes without breaking.
- Color
- Odor
- Texture (what it feels like)
- Temperature
- Mass
- Volume
- Solubility
- Thermal Conductivity
Temperature: the amount of kinetic energy (energy of motion) the particles in a substance has

Boiling Point: temperature at which a liquid changes to a gas
-
- still a physical property, because the identity of the matter is the same
- opposite is the Dew Point
-
-
- example: water vapor...or liquid water...or water ice---all still water
-
-
Melting Point: temperature at which a solid becomes a liquid
-
- Identity of the matter is still the same (ice is still water)
- Opposite is the Freezing point
Malleability: The ability to be pounded into thin sheets
-
- Example: Aluminum can be pounded to make foil
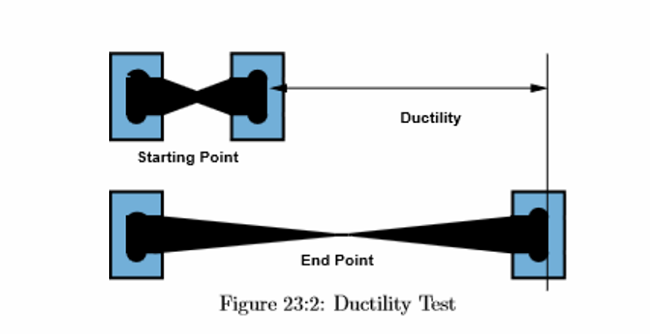
Ductility: ability to be pulled or drawn into wire
-
-
- Example: copper wiring
-
Solubility: ability to dissolve in another substance
-
- Example: salt and sugar dissolve in water
- Three ways to increase solubility
- Increase temperature (increase energy)
- Make particles smaller (increase surface area exposed)
- Mix the solution (reduce clumping)
Thermal Conductivity: ability of a material to transmit heat
-
- Example: plastic has low thermal conductivity, so won't burn
- Example: metals have high conductivity, so burn hand
:max_bytes(150000):strip_icc()/mass-volume-balance-56a12dc53df78cf772682cd0.jpg)
Matter is anything that has a mass and takes up space (volume).
- Mass: amount of matter in a substance
- measured on a triple beam balance
- measured in grams or kilograms
-
-
-
-
-
-
-
-
-
-
-
-
- Volume: amount of space a substance takes up
- milliliters (mL), Liters (L), or cubic centimeters (cc or cm3)
-
-
-
-
-
-
-
-
-
-
-

How to measure volume:
- For regular shapes:
- Volume = length x width x height
- slightly different equations for different shapes
- Volume = length x width x height
2. For liquids:
-
- Measure in a graduated cylinder
- Always look at the bottom of the meniscus
3. For gases:
-
- volume of a gas is the same as the container it is in
4. For irregular objects:
-
- Displacement method: volume of object is equal to the change in a volume of water it is placed in (how much water is "displaced")
Density: the amount of matter in a certain amount of space
-
- reflects how tightly packed particles are
- Density = mass/volume
- mass = Density x volume
- volume=Density x mass
Chemical Properties
Chemical Property: property of matter that can only be observed by changing the identity of the substance
- Can be observed with senses, but is more difficult than observing physical properties
- observing chemical properties causes the substance to change

Examples:
- Flammability (ability to burn)
- Corrosive (ability to damage/ eat away at substances)
- Reactivity with Oxygen (Oxidation = Rusting)
- molecular formula is always a chemical property
-
- the chemical make-up of matter is a chemical property
:max_bytes(150000):strip_icc()/GettyImages-548553969-56a134395f9b58b7d0bd00df.jpg)
- the chemical make-up of matter is a chemical property
Intrinsic vs Extrinsic Properties
Intrinsic Property: Property that does NOT depend on the amount present
- has the same property no matter how much is there
- intrinsic properties are determined by the structure of the molecule
- Examples:
- Color
- Texture
- Boiling point
- Density
- Malleability
Extrinsic Property: Property that DOES depend on the amount present
- varies with the amount of material present
- extrinsic properties are determined by the number of particles, not how they are arranged
- Examples:
- Mass
- Volume
- ALL chemical properties are intrinsic (does not matter how much there is)
- Physical properties may be intrinsic or extrinsic
Properties Determine Function
A substance’s properties determine its function. A function is the purpose for which something is designed. We can think of function as what or how we use something. For example, gold is an element with the property of malleability. Malleability means a substance is very flexible; it can be shaped into many different forms. Gold is used as a major source for jewelry because it can be formed into many different shapes.
Putting It Together
- Explain how your understanding of the properties of matter has changed.
- Think of another phenomenon that applies the concept of properties.
- Explain why there are different uses for these substances based on what you have learned in this section.

