Periodic Table Worksheet
- Due Feb 4, 2022 at 11:59pm
- Points 30
- Questions 11
- Available until Mar 17, 2022 at 11:59pm
- Time Limit None
- Allowed Attempts Unlimited
Instructions
The Periodic Table
Periodic table: visual arrangement of elements that provides users with quick information about the elements
- Periods: horizontal rows on the table
- Groups: vertical columns on the table
Atomic symbol: 1 or 2 letter abbreviation of the element name
- allows us to write shorthand/quickly

Atomic number: number of protons in the nucleus of an atom
- Atomic number defines the element (is ALWAYS the same for given element)
- CANNOT change without changing what the element is
Ion: atom of the same element that has a different number of electrons than other atoms of that element
- Atoms are usually neutral (same number of electrons as protons)
- Atoms can gain/ lose electrons, giving the atom a charge (+ or -)
○ +ion means lost electrons
○ -ion means gained electrons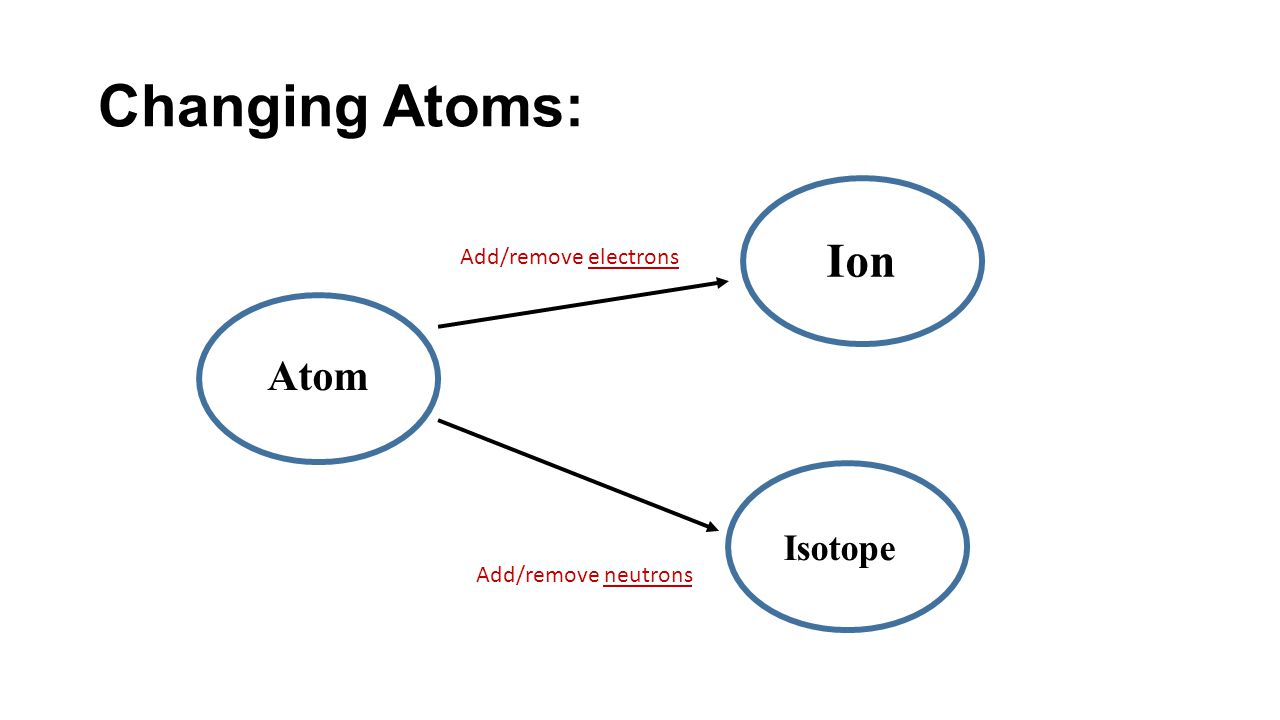
Isotope: atom of the same element that has a different number of neutrons than other atoms of that element
- Some exceptions may have more/less neutrons
Mass number= number of protons + number of neutrons
*Atomic Mass/ Atomic Weight: average mass of atoms of an element (average mass of all isotopes of an element)
Only registered, enrolled users can take graded quizzes